•Properties
•Low melting points
•Weak or No-electrolytes
Types of Carbon Atom Chains:

A straight-line
 Circular Pattern.
Circular Pattern.
Branch Pattern.
Single Bond:
Alkanes are saturated hydrocarbon which have all carbon atoms bonded by single bonds.
To name them, we have to end in "-ane" as they are Alkanes.
1: Count how many carbon is there in the LONGEST CHAIN, (usually from left to right)

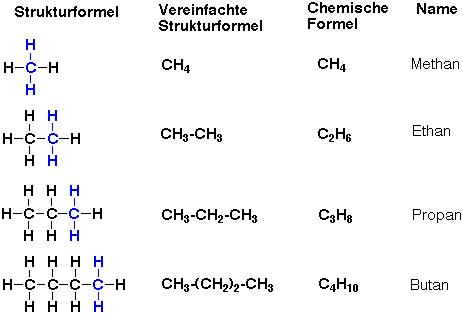
2: According to the numbers of carbon, we can record the name as a symbol " Methane, Ethane...."
3: Then, use the general formula to show the Alkane.

Double Bond (Alkene):
Generally it's the same as Alkane in the naming way, but now it's Double Bond
1: End in "-ene" instead
2: Be aware the double bonding location and name it specificly according to the information has provided!
3: Draw a double line to show it's a double bond~




Triple Bond (Alkyne):
Same method of naming to Alkene, but
1: End in "-yne" instead.
2: Draw a triple line to show it's a triple bond~




No comments:
Post a Comment