When Drawing Electron Dot and Lewis Diagrams, keep that in mind that
-the nucleus represents the symbol
-The dot represents the electrons
-There are four orbitals in each side, each orbital can only hold 2 maximum electrons
-Each shell can only hold 8 electrons
Electron dot diagrams help us to understand and represent the process of ion formation, and also illustrate that ionic bonds tend to produce full outer orbits of electron
Covalent Bonds:
Lets use water as an example, covalent bonds are composed by 2 non-metal atom as we've learned from previous classes. When we are doing it, remember:
- Fill each orbital into full shell
- Use line instead to replace the dot to represent the orbital is full
- Use the symbol to represent the nucleus.
Lets move on in to Ion bond.
Basically it's the same format, but please remember both elements are not SHARING electrons. So we use "+" to represents that they are joined together still, but not covalent bonds.
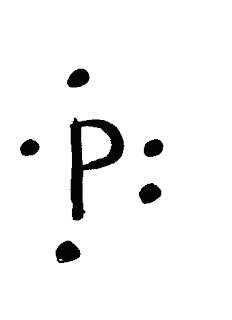



No comments:
Post a Comment