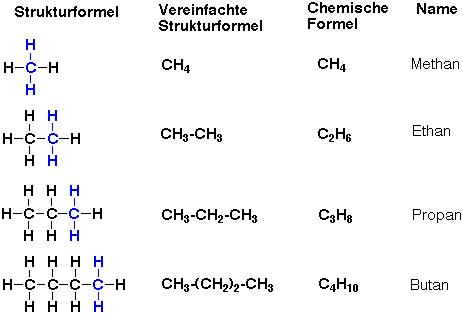
•Halides & Nitro Compounds
Naming rule is just attached to the Alkane and Alkene, but we have to follow the new prefixes:
F = Fluro
Br= Bromo
Cl = Chloro
NO3 = Nitro

Notes:
We use prefixes such as di-, tri-, tetra-.... to indicate that if there is one more Compounds.
Alcohols:
1: It contains OH- and we have to name it as the lowest number as possible (start counting it from the closest way)
2: End in - ol
3: If there is more than one OH- in it, we use prefixes such as di-, tri- to indicate it.


Aldehydes and Ketones :
The difference between aldehydes and ketones is that
1: Aldehydes contains double bond Oxygen at the end of its chain
2: Ketones contains double bond Oxygen at the MIDDLE of its chain
Aldehydes:
1: End in "-al"
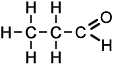


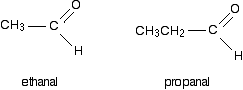
"Ketones"
1: End in "-one"